Клинический разбор в общей медицине №12 2025
Хазем Мухаммад Альмасареи4
1 Кафедра патологической анатомии, Медицинский городок Бурджил, Абу-Даби, ОАЭ;
2 Кафедра внутренних болезней, Больница Мадинат Зайед, Регион Аль-Дафра, ОАЭ;
3 Кафедра патологической анатомии, Медицинский городок Бурджил, Абу-Даби, ОАЭ;
4 Кафедра диагностической и интервенционной радиологии, Больница Мадинат Зайед, Регион Аль-Дафра, ОАЭ
Аннотация
Введение. Выбухающая дерматофибросаркома – это редкая локально агрессивная саркома мягких тканей с высокой частотой рецидивов. Трансформация в фибросаркому при выбухающей дерматофибросаркоме связана с повышением метастатического потенциала и худшим прогнозом. Лечение обычно предполагает широкое иссечение с отступом от краев, однако в случаях рецидивов и метастазирования необходимо использовать мультидисциплинарный подход, в том числе лучевую терапию и системное лечение.
Клинический случай. Представлено описание случая 37-летней пациентки с рецидивом выбухающей дерматофибросаркомы в задней грудной стенке слева. Она перенесла несколько операций по иссечению опухоли в Египте (в 2017 г., 2019 г. и 2022 г.), после которых имел место рецидив, в связи с чем в 2022 г. потребовались широкое иссечение опухоли и адъювантная лучевая терапия (50 Гр, 25 фракций). В октябре 2023 г. обследование выявило рецидив – образование размером 10×8 см, проникающее в заднюю грудную стенку.
Магнитно-резонансная томография подтвердила поражение нижележащих ребер и межреберных мышц. В ноябре 2023 г. пациентке выполнили обширную резекцию и реконструктивно-пластическую операцию, патологоанатомическое исследование подтвердило трансформацию в фибросаркому при выбухающей дерматофибросаркоме 3-й степени злокачественности (14×13×6 см) с негативными краями резекции. В послеоперационном периоде после заживления раны на 80% была запланирована адъювантная AIM-химиотерапия. Однако в ноябре 2024 г. томография выявила новое образование размером 8,8×9×8 см в головке поджелудочной железы, распространяющееся на верхнюю брыжеечную вену. Биопсия подтвердила рецидив выбухающей дерматофибросаркомы. Пациентке выполнили эндоскопическую ретроградную холангиопанкреатографию и стентирование, после чего ее обследовали на предмет дальнейшего хирургического вмешательства и лечения рака.
Заключение. Представленный случай демонстрирует агрессивный характер трансформации в фибросаркому при многоочаговом рецидиве выбухающей дерматофибросаркомы, обусловливающий необходимость наблюдения и применения мультидисциплинарного подхода к лечению. Прогрессирование заболевания, несмотря на повторные иссечения и курсы адъювантной терапии, подчеркивает необходимость применения новых терапевтических стратегий для улучшения долгосрочных результатов при выбухающей дерматофибросаркоме высокой степени злокачественности.
Ключевые слова: выбухающая дерматофибросаркома, трансформация в фибросаркому, рецидив саркомы, опухоль грудной стенки, метастазы в поджелудочной железе.
Для цитирования: Равия Мубарак Мохамед, Ашраф Альаккад, Бабита Алингал Мохаммед, Хазем Мухаммад Альмасареи. Многоочаговый рецидив выбухающей дерматофибросаркомы с трансформацией в высокодифференцированную фибросаркому: клинический случай. Clinical review for general practice. 2025; 6 (12): 37–43 (In Russ.). DOI: 10.47407/kr2025.6.12.00728
Introduction
Dermatofibrosarcoma protuberans is a type of skin cancer that tends to be locally aggressive [1]. It recurs in the same area but doesn't usually spread to other parts of the body [2]. Taylor first recognized this tumor in 1890, and then Darrier described it in 1924, but Hoffman coined the term "dermatofibrosarcoma protuberans" in 1925 [3, 4]. Reports indicate that it affects various body surfaces, primarily the trunk (42–72%), then the extremities (16–30%), and less frequently the head and neck (10–16%) [2]. Even though it makes up less than 0.1% of all malignant neoplasms, it is actually the most common skin sarcoma, accounting for nearly 1% of all soft tissue sarcomas, over 1% of all malignant tumors in the head and neck, and 7% of all head and neck sarcomas [5].
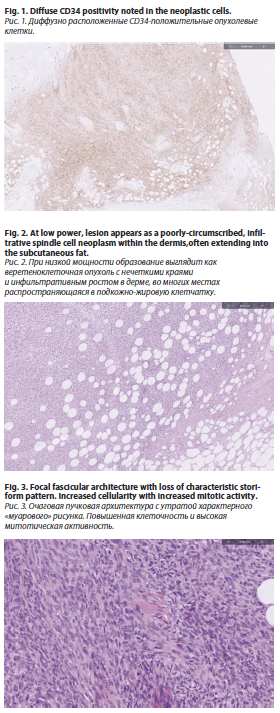
Patients with the fibrosarcomatous variant have higher recurrence rates, and the severity of cancer tends to increase with multiple recurrences [6]. This lesion is characterized by a storiform growth pattern of spindle cells found in the deep dermis [6]. The COL1A1-PDGFb fusion gene appears in about 85–90% of the cases [7]. The epidermis above DFSP appears thinner and might be separated from the tumor by a Grenz zone, based on histological examination [8]. Dermatofibrosarcoma protuberans begins as a nodule or an unclear dermal plaque that spreads into the subcutis or occasionally into skeletal muscle, showing a distinctive "honeycomb" pattern [5]. The epidermis typically isn't affected [5]. The immunohistochemical analysis shows CD34 positivity, which indicates the development of dendritic cells (fig. 1) [5]. In this case, the pancreatic lesion represents an exceptionally rare metastatic site for DFSP, likely via hematogenous spread. This highlights the need for systemic evaluation even in tumors historically known for local aggressiveness.Myoid nodules are observed more often in the fibrosarcomatous variant of DFSP [9].
About 85-90% of all DFSPs are considered low-grade tumors [10]. A small portion, about 10-15%, includes a high-grade fibrosarcoma component [10]. This transformation, which appears in over 5% of tumor volume, is marked by a greater likelihood of local relapse and distant metastasis [11]. Complete surgical resection is considered the best treatment for local DFSP [12]. However, the minimum resection margin required for local control is still not clearly defined. It's really challenging to achieve local control of the tumor [13]. For instance, after regular surgery, DFSP comes back in about 20% of cases, but with Mohs surgery, the recurrence rates drop to less than 1% [14].
Additionally, the unique villous pattern that extends into the subcutaneous fat, fascia, and muscles, while still keeping healthy tissue intact during resection, poses a significant surgical challenge (fig. 2) [15]. If complete excision isn't achieved, it can result in local recurrence [15]. This case report presents a case of recurrent dermatofibrosarcoma protuberans with high-grade fibro sarcomatous transformation.
Case presentation
A 37-year-old female patient with a history of sarcoma involving the left posterior chest wall presented for ongoing management of recurrent disease. She underwent surgical resection of the sarcoma in Egypt in 2017, 2019, and 2022. Despite multiple resections, the patient experienced tumor recurrence. Plans were made for wide local excision of the recurrent tumor, including chest wall resection involving the ribs, with subsequent plastic reconstruction.
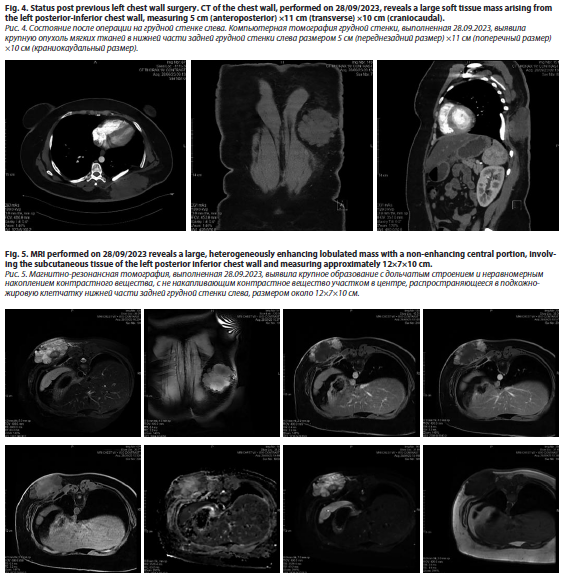
The patient had a history of multiple local recurrences of dermatofibrosarcoma protuberans (DFSP) since 2014. In 2019, PET/CT revealed metastatic lesions in the lungs, for which she underwent metastatectomies. In 2022, a local recurrence at the previous left posterior chest wall surgery site led to a wide local excision, followed by adjuvant radiation therapy (50 Gy in 25 fractions), completed on August 1, 2022 (fig. 4).
Later on October 13, 2023, the patient was evaluated in the plastic surgery clinic, following a referral from the thoracic surgery department for further assessment and surgical planning. The multidisciplinary team scheduled the excision and reconstruction surgery for November 23, 2023. On examination, a recurrent sarcoma measuring approximately 10×8 cm was noted on the left posterior trunk. MRI of the chest with contrast revealed a large, heterogeneously enhancing lobulated mass within the subcutaneous tissue of the left posteroinferior chest wall, measuring approximately 12×7×10 cm (fig 5). Abnormal signal intensity with some enhancement was observed in the posterior aspects of the underlying ribs (7–9) and adjacent intercostal muscles, but there was no evidence of spinal extension.
The most recent resection on November 23, 2023, confirmed a unifocal tumor measuring 14×13×6 cm, identified as Grade 3 DFSP with high-grade fibrosarcomatous transformation. Pathology findings included a mitotic rate of 20 mitoses per 10 high-power fields, 30–40% necrosis, negative margins, and no evidence of lymphovascular invasion (pT3). Histological analysis conducted on 23 November 2023 showed an infiltrative spindle cell proliferation with storiform architecture and CD34 positivity with adjacent areas of fascicular and herringbone growth pattern and extensive necrosis (fig. 3).
 On March 8, 2024, the patient underwent a split-thickness skin graft procedure. By August 27, 2024, plastic surgery was completed without major complications. The patient was doing well and underwent daily wound dressings. Upon review with the Doctor, it was noted that wound healing was at 80%, and further assessment was planned for the following day to determine the timing of chemotherapy initiation. The oncology team discussed the AIM chemotherapy regimen (doxorubicin, ifosfamide, mesna) with the patient, explaining its rationale due to the high-grade transformation and systemic spread. The preliminary plan was to commence chemotherapy on April 15, 2024, pending wound healing progress. A MUGA scan and port placement were ordered. Additionally, the patient was referred to gynecology for fertility preservation counseling before initiating chemotherapy due to the potential risk of infertility, as she is gravida 0. She was scheduled for a follow-up on April 15, 2024, to reassess wound healing and finalize chemotherapy initiation.
On March 8, 2024, the patient underwent a split-thickness skin graft procedure. By August 27, 2024, plastic surgery was completed without major complications. The patient was doing well and underwent daily wound dressings. Upon review with the Doctor, it was noted that wound healing was at 80%, and further assessment was planned for the following day to determine the timing of chemotherapy initiation. The oncology team discussed the AIM chemotherapy regimen (doxorubicin, ifosfamide, mesna) with the patient, explaining its rationale due to the high-grade transformation and systemic spread. The preliminary plan was to commence chemotherapy on April 15, 2024, pending wound healing progress. A MUGA scan and port placement were ordered. Additionally, the patient was referred to gynecology for fertility preservation counseling before initiating chemotherapy due to the potential risk of infertility, as she is gravida 0. She was scheduled for a follow-up on April 15, 2024, to reassess wound healing and finalize chemotherapy initiation.
The patient was evaluated on multiple occasions, with vital signs recorded on March 26 and March 27, 2024. On March 27 at 11:15 AM UAE time, the patient's temperature was 36.7 °C (tympanic), with a peripheral pulse rate of 88 beats per minute and a respiratory rate of 18 breaths per minute. Blood pressure measured via machine was 129/78 mmHg, with a mean arterial pressure of 95 mmHg. Oxygen saturation was recorded at 99%. The previous day, at 14:29 PM, the patient's temperature was slightly higher at 36.8°C, with a pulse rate of 98 beats per minute and an identical respiratory rate of 18 breaths per minute. Blood pressure at that time was slightly elevated at 132/86 mmHg, with a mean arterial pressure of 101 mmHg. Oxygen saturation was 98%.
Anthropometric measurements on March 27 indicated that the patient had a height of 165 cm and a weight of 111.5 kg, resulting in a body mass index (BMI) of 40.96 kg/m2, classifying them as having severe obesity. The body surface area (BSA) was calculated at 2.26 m2, with an ideal body weight of 56.91 kg and an adjusted body weight of 78.75 kg. These measurements highlight a significant discrepancy between actual and ideal body weight, emphasizing the need for weight management interventions. The patient’s obesity was considered in planning for chemotherapy dosing and potential wound healing delays.
During the physical examination, the patient was alert and oriented, with no acute distress. The eyes showed equal, round, and reactive pupils to light, with intact extraocular movements and normal conjunctiva. Head, ear, nose, and throat (HENT) examination revealed a normocephalic appearance, moist oral mucosa, and no signs of pharyngeal erythema. The neck was supple, and non-tender, with no carotid bruits, jugular venous distention, or lymphadenopathy.
Respiratory examination demonstrated clear lungs to auscultation, non-labored respirations, equal breath sounds, and symmetrical chest wall expansion. Cardiovascular assessment showed a normal heart rate and regular rhythm, with no murmurs, gallops, or edema. The gastrointestinal examination revealed a soft, non-tender, and non-distended abdomen with normal bowel sounds and no organomegaly. The genitourinary examination was unremarkable, with no costovertebral angle tenderness.
Neurologically, the patient was alert and oriented, with normal sensory and motor function and no focal deficits. Cognitive and speech assessment indicated clear and coherent speech, with functional cognition intact. The psychiatric evaluation showed a cooperative patient with an appropriate mood and affect, as well as normal judgment.
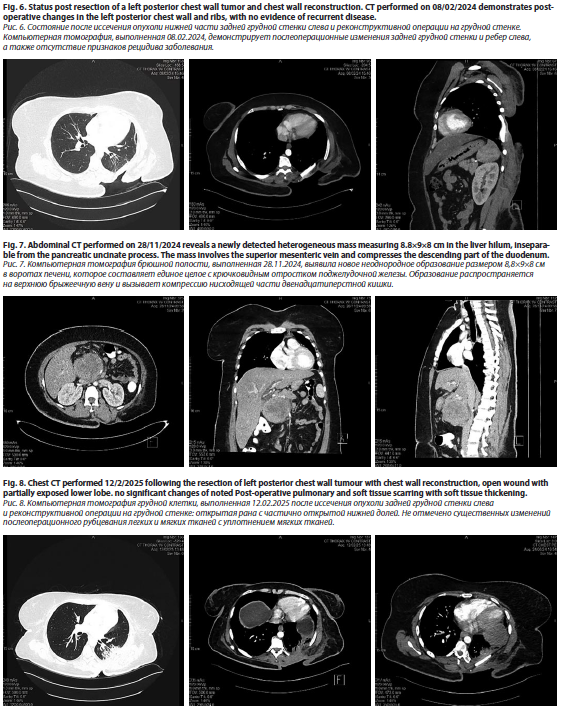
The patient had a known history of G3 dermatofibrosarcoma protuberans with high-grade fibrosarcomatous transformation, status post resection (fig 6). Wound healing was noted to be at 80%, and further evaluation with Doctors was planned for the following day to determine the timeline for initiating chemotherapy. The patient was counseled regarding the AIM regimen in the adjuvant setting, consisting of six cycles, and consent was obtained for treatment initiation on April 15, 2024, pending wound healing status.
To facilitate chemotherapy, a Multi-Gated Acquisition (MUGA) scan and PORT placement have been ordered. Additionally, the patient was referred to gynecology to discuss fertility preservation options prior to chemotherapy, given the associated risk of infertility and her G0 status.
The patient was scheduled for chemotherapy starting April 15, 2024, with a five-day regimen every three weeks for six cycles. Anticipated side effects included immune suppression, fatigue, nausea, vomiting, and the potential need for medical leave during treatment. The patient was reassessed at the next visit to confirm wound healing progress before proceeding with chemotherapy.
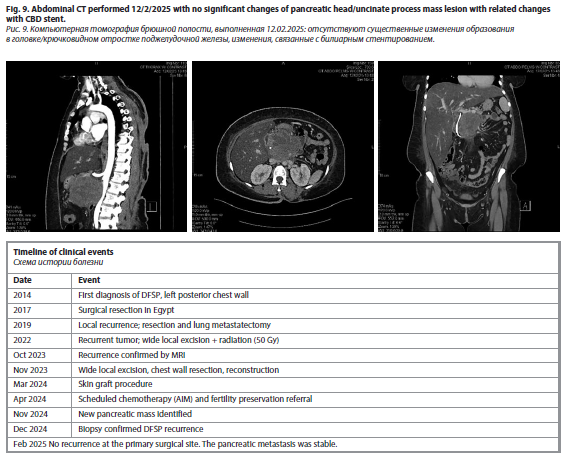
Later, a CT scan of the chest, abdomen, and pelvis performed on 20/11/24 revealed a normal abdominal wall and liver with no detectable focal lesions (fig. 7–9). The gallbladder had been surgically removed, and there was mild dilation of the intrahepatic biliary radicles. A new interval finding of a heterogeneous mass measuring 8.8×9×8 cm was noted in the liver hilum, inseparable from the pancreatic uncinate process, involving the superior mesenteric vein and compressing the descending part of the duodenum. There was mild prominence of the pancreatic duct, while the pancreas itself appeared normal in size and density. The spleen, adrenal glands, and kidneys were normal, with no focal lesions or signs of perinephric fluid accumulation. The urinary bladder was underfilled, with an inflated Foley's catheter balloon. The uterus was anteverted with a tiny subserosal myoma at its fundus, while the right and left ovaries measured 49×30 mm and 30×20 mm, respectively. Minimal free pelvic fluid was noted on the left side. The gastrointestinal tract showed no abnormal wall thickening, and no abnormally sized or appearing lymph nodes were detected. The abdominal vessels were normal, without stenotic plaques, and the mesentery and peritoneum appeared unremarkable. No signs of bone destruction were observed.
A new interval finding of a pancreatic head mass was identified, involving the superior mesenteric vein and compressing the descending duodenum. There was no definitive sign of local recurrence of the previously resected left posterior chest wall tumor. An ERCP was performed on 29/11/24 with the placement of a plastic stent. A biopsy review on 11/12/24 confirmed that the pancreatic head mass exhibited features favoring dermatofibrosarcoma. She was scheduled for evaluation by the plastic surgery team to assess a wound defect on the upper back. After the plastic surgery, the patient recovered completely.
Discussion
In the present case, a 37-year-old female was found to have recurrent dermatofibrosarcoma protuberans (DFSP) at the left posterior chest wall area accompanied by several local recurrences and the metastatic spread to the lungs and pancreas. Simultaneous to these resections between 2014 and 2023, the patient underwent wide local excision, adjuvant radiation therapy, and intended chemotherapy, but the disease recurred. The most recent findings include a pancreatic mass confirmed as DFSP with an aggressive disease course and fibrosarcomatous transformation.
DFSP is a skin tumor that comes from the dermis and is known for its slow growth that spreads into surrounding tissues [16]. It often recurs after surgery but usually doesn't spread to other parts of the body [17]. The way the tumor looks in a clinical setting really depends on how advanced the disease is [17]. At first, it looks pink-to-bluish-red patch on the skin that can be trophic or sclerotic [17]. Over time, it changes into a lumpy nodular mass and eventually becomes an ulcerative, bleeding tumor that sticks out [18]. It feels like it's just on the surface and moves around when touched since it's attached to the skin above, but not to the tissues below [19]. Unfortunately, in the later stage of the tumor, there may be fixation to deeper structures like fascia and muscle [20]. Scalp fixation due to periosteal attachment might happen in the early stages. Telangiectasia can be seen on the surface or around the edges [21].
The tumor grows slowly, so it can take anywhere from weeks to years to develop [21]. It's pretty common for there to be a delay in diagnosis and for the initial lesion to be misdiagnosed, mainly because there aren't any symptoms present [21].
Immunohistochemical staining shows strong positivity for CD34 (sensitivity 84–100%) and vimentin while being negative for CD44, S-100, staining. Factor XIIIa staining. The positivity of the last marker and stromelysin 3 (ST3) is helpful for distinguishing benign fibrous histiocytoma (dermatofibroma) [22]. In our case, Histological analysis showed an infiltrative spindle cell proliferation with storiform architecture and CD34 positivity with adjacent areas of fascicular and herringbone growth pattern and extensive necrosis (fig. 3).
The literature review shows that DFSP rarely metastasizes, and in case of distant spread, it involves the lungs most commonly. As an example, Lombart et al. report a case of recurrent DFSP of the chest wall that metastasized to the lungs and liver [23]. In contrast to our case, metastases from squamous cell carcinomas of the skin usually occur in the pulmonary system, whereas metastases to the pancreas are a highly unusual site. There are also cases similar to Kimmel et al. in which there was a local recurrence of the disease with no distant metastases despite multiple resections and adjuvant radiotherapy in a DFSP with fibrosarcomatous change [24]. Nikolaus et al. also described a case of 45-year-old male with DFSP of the trunk, treated with wide local excision and adjuvant imatinib therapy for PDGFB overexpression [15].
DFSPs have a really strong tendency to invade the nearby tissue. The typical treatment method for this tumor involves a wide and deep local excision (WLE), which also includes the underlying fascia. In a study observing 159 cases, it was found that even though 99% of the patient’s group had complete surgical resection, the pathologic review revealed that only 93 patients (58%) had negative microscopic margins [25]. Lindner et al. found that removing healthy tissues in a circumferential range of 2.5–3.5 cm helped improve local control of the disease [26]. Most people seem to agree that having 3–5 cm of lateral and deep margins is enough for controlling the disease locally.
Conclusion
This case highlights the challenges in surgery for recurrent DFSP with fibrosarcomatous transformation. Pancreatic metastasis, the unique presentation, and the aggressive recurrence pattern underscore the need for vigilant long-term follow-up and consideration for systemic therapies beyond surgery and radiation. Optimal treatment strategies for high-risk DFSP patients needs further studies.
Conflict of interests. The authors declare that there is not conflict of interests.
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
The contribution of the authors. Ashraf ALakkad made a major contribution to the development of the concept of the article with writing and editing the case report. Rawia Mubarak Mohamed agreed to take responsibility for all aspects of the case report. Hazem Muhammad Almasarei was responsible for the radiological analysis and its interpretation in the article. Babitha Alingal Mohammed assisted in the analysis of histopathology images. All the authors approved the final version of the article.
Вклад авторов. Ашраф Альаккад внес основной вклад в разработку концепции статьи, а также написал и отредактировал описание клинического случая. Равия Мубарак Мохамед согласилась взять на себя ответственность за все аспекты представленного описания клинического случая. Хазем Мухаммад Альмасареи отвечал за радиологические исследования и интерпретацию их результатов в представленной статье. Бабита Алингал Мохаммед помогла проанализировать гистологические изображения. Все авторы одобрили финальную версию статьи.
Список литературы доступен на сайте журнала https://klin-razbor.ru/
The list of references is available on the journal‘s website https://klin-razbor.ru/
Информация об авторах
Information about the authors
Равия Мубарак Мохамед – доктор медицины, бакалавр медицины и бакалавр хирургии, член Королевской коллегии патологов Австралии, зав. кафедрой, консультант по патологической анатомии, Burjeel Holdings, ОАЭ. ORCID: 0009-0000-2320-4063
Dr. Rawia Mubarak Mohamed – MD, MBBS, FRCPA Head of Department, Consultant Anatomic Pathology, Burjeel Holdings, UAE. ORCID: 0009-0000-2320-4063
Ашраф Альаккад – доктор медицины, врач-терапевт, каф. внутренних болезней, руководитель программы управления фармакоэпидемиологическими исследованиями, Больница Мадинат Зайед, ОАЭ. ORCID: 0000-0002-4083-2800; Scopus Author ID: 60052817400; Web Of Science Researcher ID: AEW-9201-2022
Dr. Ashraf ALakkad – MD, Internist, Department of Internal Medicine, Chair of Antimicrobial Stewardship Program, Madinat Zayed Hospital, UAE. ORCID: 0000-0002-4083-2800. Scopus Author ID: 60052817400; Web Of Science Researcher ID: AEW-9201-2022
Бабита Алингал Мохаммед – доктор медицины, дипломант Национального совета медицинских экзаменаторов, член Национальной академии медицинских наук, член Королевской коллегии патологов (Великобритания), специалист по патологической анатомии и клинической патологии в медицинском городке Шейха Шахбута (SSMC) в Абу-Даби. ORCID: 0009-0003-3234-0384
Dr. Babitha Alingal Mohammed – MD, D.N.B., M.N.A.M.S., F.R.C.Path. (UK), is an anatomic and clinical pathology specialist at Sheikh Shakhbout Medical City (SSMC) in Abu Dhabi. ORCID: 0009-0003-3234-0384
Хазем Мухаммад Альмасареи – доктор медицины, консультант по диагностической и интервенционной радиологии, кафедра диагностической и интервенционной радиологии, Больница Мадинат Зайед, ОАЭ. ORCID: 0000-0001-9572-3719
Dr. Hazem M. Almasarei – MD, Consultant diagnostic and interventional radiology, Department of diagnostic and interventional radiology, Madinat Zayed Hospital, UAE. ORCID: 0000-0001-9572-3719
Поступила в редакцию: 06.10.2025
Поступила после рецензирования: 14.10.2025
Принята к публикации: 16.10.2025
Received: 06.10.2025
Revised: 14.10.2025
Accepted: 16.10.2025
Клинический разбор в общей медицине №12 2025
Multisite recurrence of dermatofibrosarcoma protuberans with high-grade fibrosarcomatous transformation: a case report
Номера страниц в выпуске:37-43
Abstract
Background. Dermatofibrosarcoma protuberans (DFSP) is a rare, locally aggressive soft tissue sarcoma with a high recurrence rate. The fibrosarcomatous transformation of DFSP (FS-DFSP) is associated with increased metastatic potential and poorer prognosis. Management typically involves wide surgical excision with clear margins, but recurrent and metastatic cases require a multidisciplinary approach, including radiation therapy and systemic treatment.
Case presentation. We report a 37-year-old female with a history of recurrent DFSP involving the left posterior chest wall. She underwent multiple surgical resections in Egypt (2017, 2019, 2022), with a subsequent recurrence requiring wide local excision and adjuvant radiation therapy (50 Gy in 25 fractions) in 2022. In October 2023, evaluation revealed a recurrent 10×8 cm mass infiltrating the posterior chest wall. MRI confirmed the involvement of the underlying ribs and intercostal muscles. She underwent extensive resection with plastic reconstruction in November 2023, with pathology confirming Grade 3 FS-DFSP (14×13×6 cm) with negative margins. Postoperatively, wound healing progressed to 80%, and adjuvant AIM chemotherapy was planned. However, in November 2024, imaging detected a new 8.8×9×8 cm mass in the pancreatic head involving the superior mesenteric vein. Biopsy confirmed DFSP recurrence. The patient underwent ERCP with stent placement and was re-evaluated for further surgical and oncological management.
Conclusion. This case highlights the aggressive nature of FS-DFSP with multisite recurrence, necessitating ongoing surveillance and a multidisciplinary treatment approach. Despite repeated resections and adjuvant therapies, disease progression underscores the need for novel therapeutic strategies to improve long-term outcomes in high-grade DFSP.
Keywords: Dermatofibrosarcoma protuberans, fibrosarcomatous transformation, recurrent sarcoma, chest wall tumor, pancreatic metastasis.
For citation: Rawia Mubarak Mohamed, Ashraf ALakkad, Babitha Alingal Mohammed, Hazem Muhammad ALmasarei. Multisite recurrence of dermatofibrosarcoma protuberans with high-grade fibrosarcomatous transformation: a case report. Clinical review for general practice. 2025; 6 (12): 37–43 (In Russ.). DOI: 10.47407/kr2025.6.12.00728
Background. Dermatofibrosarcoma protuberans (DFSP) is a rare, locally aggressive soft tissue sarcoma with a high recurrence rate. The fibrosarcomatous transformation of DFSP (FS-DFSP) is associated with increased metastatic potential and poorer prognosis. Management typically involves wide surgical excision with clear margins, but recurrent and metastatic cases require a multidisciplinary approach, including radiation therapy and systemic treatment.
Case presentation. We report a 37-year-old female with a history of recurrent DFSP involving the left posterior chest wall. She underwent multiple surgical resections in Egypt (2017, 2019, 2022), with a subsequent recurrence requiring wide local excision and adjuvant radiation therapy (50 Gy in 25 fractions) in 2022. In October 2023, evaluation revealed a recurrent 10×8 cm mass infiltrating the posterior chest wall. MRI confirmed the involvement of the underlying ribs and intercostal muscles. She underwent extensive resection with plastic reconstruction in November 2023, with pathology confirming Grade 3 FS-DFSP (14×13×6 cm) with negative margins. Postoperatively, wound healing progressed to 80%, and adjuvant AIM chemotherapy was planned. However, in November 2024, imaging detected a new 8.8×9×8 cm mass in the pancreatic head involving the superior mesenteric vein. Biopsy confirmed DFSP recurrence. The patient underwent ERCP with stent placement and was re-evaluated for further surgical and oncological management.
Conclusion. This case highlights the aggressive nature of FS-DFSP with multisite recurrence, necessitating ongoing surveillance and a multidisciplinary treatment approach. Despite repeated resections and adjuvant therapies, disease progression underscores the need for novel therapeutic strategies to improve long-term outcomes in high-grade DFSP.
Keywords: Dermatofibrosarcoma protuberans, fibrosarcomatous transformation, recurrent sarcoma, chest wall tumor, pancreatic metastasis.
For citation: Rawia Mubarak Mohamed, Ashraf ALakkad, Babitha Alingal Mohammed, Hazem Muhammad ALmasarei. Multisite recurrence of dermatofibrosarcoma protuberans with high-grade fibrosarcomatous transformation: a case report. Clinical review for general practice. 2025; 6 (12): 37–43 (In Russ.). DOI: 10.47407/kr2025.6.12.00728
Многоочаговый рецидив выбухающей дерматофибросаркомы с трансформацией в высокодифференцированную фибросаркому: клинический случай
Равия Мубарак Мохамед1, Ашраф Альаккад2, Бабита Алингал Мохаммед3,Хазем Мухаммад Альмасареи4
1 Кафедра патологической анатомии, Медицинский городок Бурджил, Абу-Даби, ОАЭ;
2 Кафедра внутренних болезней, Больница Мадинат Зайед, Регион Аль-Дафра, ОАЭ;
3 Кафедра патологической анатомии, Медицинский городок Бурджил, Абу-Даби, ОАЭ;
4 Кафедра диагностической и интервенционной радиологии, Больница Мадинат Зайед, Регион Аль-Дафра, ОАЭ
Аннотация
Введение. Выбухающая дерматофибросаркома – это редкая локально агрессивная саркома мягких тканей с высокой частотой рецидивов. Трансформация в фибросаркому при выбухающей дерматофибросаркоме связана с повышением метастатического потенциала и худшим прогнозом. Лечение обычно предполагает широкое иссечение с отступом от краев, однако в случаях рецидивов и метастазирования необходимо использовать мультидисциплинарный подход, в том числе лучевую терапию и системное лечение.
Клинический случай. Представлено описание случая 37-летней пациентки с рецидивом выбухающей дерматофибросаркомы в задней грудной стенке слева. Она перенесла несколько операций по иссечению опухоли в Египте (в 2017 г., 2019 г. и 2022 г.), после которых имел место рецидив, в связи с чем в 2022 г. потребовались широкое иссечение опухоли и адъювантная лучевая терапия (50 Гр, 25 фракций). В октябре 2023 г. обследование выявило рецидив – образование размером 10×8 см, проникающее в заднюю грудную стенку.
Магнитно-резонансная томография подтвердила поражение нижележащих ребер и межреберных мышц. В ноябре 2023 г. пациентке выполнили обширную резекцию и реконструктивно-пластическую операцию, патологоанатомическое исследование подтвердило трансформацию в фибросаркому при выбухающей дерматофибросаркоме 3-й степени злокачественности (14×13×6 см) с негативными краями резекции. В послеоперационном периоде после заживления раны на 80% была запланирована адъювантная AIM-химиотерапия. Однако в ноябре 2024 г. томография выявила новое образование размером 8,8×9×8 см в головке поджелудочной железы, распространяющееся на верхнюю брыжеечную вену. Биопсия подтвердила рецидив выбухающей дерматофибросаркомы. Пациентке выполнили эндоскопическую ретроградную холангиопанкреатографию и стентирование, после чего ее обследовали на предмет дальнейшего хирургического вмешательства и лечения рака.
Заключение. Представленный случай демонстрирует агрессивный характер трансформации в фибросаркому при многоочаговом рецидиве выбухающей дерматофибросаркомы, обусловливающий необходимость наблюдения и применения мультидисциплинарного подхода к лечению. Прогрессирование заболевания, несмотря на повторные иссечения и курсы адъювантной терапии, подчеркивает необходимость применения новых терапевтических стратегий для улучшения долгосрочных результатов при выбухающей дерматофибросаркоме высокой степени злокачественности.
Ключевые слова: выбухающая дерматофибросаркома, трансформация в фибросаркому, рецидив саркомы, опухоль грудной стенки, метастазы в поджелудочной железе.
Для цитирования: Равия Мубарак Мохамед, Ашраф Альаккад, Бабита Алингал Мохаммед, Хазем Мухаммад Альмасареи. Многоочаговый рецидив выбухающей дерматофибросаркомы с трансформацией в высокодифференцированную фибросаркому: клинический случай. Clinical review for general practice. 2025; 6 (12): 37–43 (In Russ.). DOI: 10.47407/kr2025.6.12.00728
Introduction
Dermatofibrosarcoma protuberans is a type of skin cancer that tends to be locally aggressive [1]. It recurs in the same area but doesn't usually spread to other parts of the body [2]. Taylor first recognized this tumor in 1890, and then Darrier described it in 1924, but Hoffman coined the term "dermatofibrosarcoma protuberans" in 1925 [3, 4]. Reports indicate that it affects various body surfaces, primarily the trunk (42–72%), then the extremities (16–30%), and less frequently the head and neck (10–16%) [2]. Even though it makes up less than 0.1% of all malignant neoplasms, it is actually the most common skin sarcoma, accounting for nearly 1% of all soft tissue sarcomas, over 1% of all malignant tumors in the head and neck, and 7% of all head and neck sarcomas [5].
Patients with the fibrosarcomatous variant have higher recurrence rates, and the severity of cancer tends to increase with multiple recurrences [6]. This lesion is characterized by a storiform growth pattern of spindle cells found in the deep dermis [6]. The COL1A1-PDGFb fusion gene appears in about 85–90% of the cases [7]. The epidermis above DFSP appears thinner and might be separated from the tumor by a Grenz zone, based on histological examination [8]. Dermatofibrosarcoma protuberans begins as a nodule or an unclear dermal plaque that spreads into the subcutis or occasionally into skeletal muscle, showing a distinctive "honeycomb" pattern [5]. The epidermis typically isn't affected [5]. The immunohistochemical analysis shows CD34 positivity, which indicates the development of dendritic cells (fig. 1) [5]. In this case, the pancreatic lesion represents an exceptionally rare metastatic site for DFSP, likely via hematogenous spread. This highlights the need for systemic evaluation even in tumors historically known for local aggressiveness.Myoid nodules are observed more often in the fibrosarcomatous variant of DFSP [9].
About 85-90% of all DFSPs are considered low-grade tumors [10]. A small portion, about 10-15%, includes a high-grade fibrosarcoma component [10]. This transformation, which appears in over 5% of tumor volume, is marked by a greater likelihood of local relapse and distant metastasis [11]. Complete surgical resection is considered the best treatment for local DFSP [12]. However, the minimum resection margin required for local control is still not clearly defined. It's really challenging to achieve local control of the tumor [13]. For instance, after regular surgery, DFSP comes back in about 20% of cases, but with Mohs surgery, the recurrence rates drop to less than 1% [14].
Additionally, the unique villous pattern that extends into the subcutaneous fat, fascia, and muscles, while still keeping healthy tissue intact during resection, poses a significant surgical challenge (fig. 2) [15]. If complete excision isn't achieved, it can result in local recurrence [15]. This case report presents a case of recurrent dermatofibrosarcoma protuberans with high-grade fibro sarcomatous transformation.
Case presentation
A 37-year-old female patient with a history of sarcoma involving the left posterior chest wall presented for ongoing management of recurrent disease. She underwent surgical resection of the sarcoma in Egypt in 2017, 2019, and 2022. Despite multiple resections, the patient experienced tumor recurrence. Plans were made for wide local excision of the recurrent tumor, including chest wall resection involving the ribs, with subsequent plastic reconstruction.
The patient had a history of multiple local recurrences of dermatofibrosarcoma protuberans (DFSP) since 2014. In 2019, PET/CT revealed metastatic lesions in the lungs, for which she underwent metastatectomies. In 2022, a local recurrence at the previous left posterior chest wall surgery site led to a wide local excision, followed by adjuvant radiation therapy (50 Gy in 25 fractions), completed on August 1, 2022 (fig. 4).
Later on October 13, 2023, the patient was evaluated in the plastic surgery clinic, following a referral from the thoracic surgery department for further assessment and surgical planning. The multidisciplinary team scheduled the excision and reconstruction surgery for November 23, 2023. On examination, a recurrent sarcoma measuring approximately 10×8 cm was noted on the left posterior trunk. MRI of the chest with contrast revealed a large, heterogeneously enhancing lobulated mass within the subcutaneous tissue of the left posteroinferior chest wall, measuring approximately 12×7×10 cm (fig 5). Abnormal signal intensity with some enhancement was observed in the posterior aspects of the underlying ribs (7–9) and adjacent intercostal muscles, but there was no evidence of spinal extension.
The most recent resection on November 23, 2023, confirmed a unifocal tumor measuring 14×13×6 cm, identified as Grade 3 DFSP with high-grade fibrosarcomatous transformation. Pathology findings included a mitotic rate of 20 mitoses per 10 high-power fields, 30–40% necrosis, negative margins, and no evidence of lymphovascular invasion (pT3). Histological analysis conducted on 23 November 2023 showed an infiltrative spindle cell proliferation with storiform architecture and CD34 positivity with adjacent areas of fascicular and herringbone growth pattern and extensive necrosis (fig. 3).
 On March 8, 2024, the patient underwent a split-thickness skin graft procedure. By August 27, 2024, plastic surgery was completed without major complications. The patient was doing well and underwent daily wound dressings. Upon review with the Doctor, it was noted that wound healing was at 80%, and further assessment was planned for the following day to determine the timing of chemotherapy initiation. The oncology team discussed the AIM chemotherapy regimen (doxorubicin, ifosfamide, mesna) with the patient, explaining its rationale due to the high-grade transformation and systemic spread. The preliminary plan was to commence chemotherapy on April 15, 2024, pending wound healing progress. A MUGA scan and port placement were ordered. Additionally, the patient was referred to gynecology for fertility preservation counseling before initiating chemotherapy due to the potential risk of infertility, as she is gravida 0. She was scheduled for a follow-up on April 15, 2024, to reassess wound healing and finalize chemotherapy initiation.
On March 8, 2024, the patient underwent a split-thickness skin graft procedure. By August 27, 2024, plastic surgery was completed without major complications. The patient was doing well and underwent daily wound dressings. Upon review with the Doctor, it was noted that wound healing was at 80%, and further assessment was planned for the following day to determine the timing of chemotherapy initiation. The oncology team discussed the AIM chemotherapy regimen (doxorubicin, ifosfamide, mesna) with the patient, explaining its rationale due to the high-grade transformation and systemic spread. The preliminary plan was to commence chemotherapy on April 15, 2024, pending wound healing progress. A MUGA scan and port placement were ordered. Additionally, the patient was referred to gynecology for fertility preservation counseling before initiating chemotherapy due to the potential risk of infertility, as she is gravida 0. She was scheduled for a follow-up on April 15, 2024, to reassess wound healing and finalize chemotherapy initiation.The patient was evaluated on multiple occasions, with vital signs recorded on March 26 and March 27, 2024. On March 27 at 11:15 AM UAE time, the patient's temperature was 36.7 °C (tympanic), with a peripheral pulse rate of 88 beats per minute and a respiratory rate of 18 breaths per minute. Blood pressure measured via machine was 129/78 mmHg, with a mean arterial pressure of 95 mmHg. Oxygen saturation was recorded at 99%. The previous day, at 14:29 PM, the patient's temperature was slightly higher at 36.8°C, with a pulse rate of 98 beats per minute and an identical respiratory rate of 18 breaths per minute. Blood pressure at that time was slightly elevated at 132/86 mmHg, with a mean arterial pressure of 101 mmHg. Oxygen saturation was 98%.
Anthropometric measurements on March 27 indicated that the patient had a height of 165 cm and a weight of 111.5 kg, resulting in a body mass index (BMI) of 40.96 kg/m2, classifying them as having severe obesity. The body surface area (BSA) was calculated at 2.26 m2, with an ideal body weight of 56.91 kg and an adjusted body weight of 78.75 kg. These measurements highlight a significant discrepancy between actual and ideal body weight, emphasizing the need for weight management interventions. The patient’s obesity was considered in planning for chemotherapy dosing and potential wound healing delays.
During the physical examination, the patient was alert and oriented, with no acute distress. The eyes showed equal, round, and reactive pupils to light, with intact extraocular movements and normal conjunctiva. Head, ear, nose, and throat (HENT) examination revealed a normocephalic appearance, moist oral mucosa, and no signs of pharyngeal erythema. The neck was supple, and non-tender, with no carotid bruits, jugular venous distention, or lymphadenopathy.
Respiratory examination demonstrated clear lungs to auscultation, non-labored respirations, equal breath sounds, and symmetrical chest wall expansion. Cardiovascular assessment showed a normal heart rate and regular rhythm, with no murmurs, gallops, or edema. The gastrointestinal examination revealed a soft, non-tender, and non-distended abdomen with normal bowel sounds and no organomegaly. The genitourinary examination was unremarkable, with no costovertebral angle tenderness.
Neurologically, the patient was alert and oriented, with normal sensory and motor function and no focal deficits. Cognitive and speech assessment indicated clear and coherent speech, with functional cognition intact. The psychiatric evaluation showed a cooperative patient with an appropriate mood and affect, as well as normal judgment.

The patient had a known history of G3 dermatofibrosarcoma protuberans with high-grade fibrosarcomatous transformation, status post resection (fig 6). Wound healing was noted to be at 80%, and further evaluation with Doctors was planned for the following day to determine the timeline for initiating chemotherapy. The patient was counseled regarding the AIM regimen in the adjuvant setting, consisting of six cycles, and consent was obtained for treatment initiation on April 15, 2024, pending wound healing status.
To facilitate chemotherapy, a Multi-Gated Acquisition (MUGA) scan and PORT placement have been ordered. Additionally, the patient was referred to gynecology to discuss fertility preservation options prior to chemotherapy, given the associated risk of infertility and her G0 status.
The patient was scheduled for chemotherapy starting April 15, 2024, with a five-day regimen every three weeks for six cycles. Anticipated side effects included immune suppression, fatigue, nausea, vomiting, and the potential need for medical leave during treatment. The patient was reassessed at the next visit to confirm wound healing progress before proceeding with chemotherapy.


Later, a CT scan of the chest, abdomen, and pelvis performed on 20/11/24 revealed a normal abdominal wall and liver with no detectable focal lesions (fig. 7–9). The gallbladder had been surgically removed, and there was mild dilation of the intrahepatic biliary radicles. A new interval finding of a heterogeneous mass measuring 8.8×9×8 cm was noted in the liver hilum, inseparable from the pancreatic uncinate process, involving the superior mesenteric vein and compressing the descending part of the duodenum. There was mild prominence of the pancreatic duct, while the pancreas itself appeared normal in size and density. The spleen, adrenal glands, and kidneys were normal, with no focal lesions or signs of perinephric fluid accumulation. The urinary bladder was underfilled, with an inflated Foley's catheter balloon. The uterus was anteverted with a tiny subserosal myoma at its fundus, while the right and left ovaries measured 49×30 mm and 30×20 mm, respectively. Minimal free pelvic fluid was noted on the left side. The gastrointestinal tract showed no abnormal wall thickening, and no abnormally sized or appearing lymph nodes were detected. The abdominal vessels were normal, without stenotic plaques, and the mesentery and peritoneum appeared unremarkable. No signs of bone destruction were observed.
A new interval finding of a pancreatic head mass was identified, involving the superior mesenteric vein and compressing the descending duodenum. There was no definitive sign of local recurrence of the previously resected left posterior chest wall tumor. An ERCP was performed on 29/11/24 with the placement of a plastic stent. A biopsy review on 11/12/24 confirmed that the pancreatic head mass exhibited features favoring dermatofibrosarcoma. She was scheduled for evaluation by the plastic surgery team to assess a wound defect on the upper back. After the plastic surgery, the patient recovered completely.
Discussion
In the present case, a 37-year-old female was found to have recurrent dermatofibrosarcoma protuberans (DFSP) at the left posterior chest wall area accompanied by several local recurrences and the metastatic spread to the lungs and pancreas. Simultaneous to these resections between 2014 and 2023, the patient underwent wide local excision, adjuvant radiation therapy, and intended chemotherapy, but the disease recurred. The most recent findings include a pancreatic mass confirmed as DFSP with an aggressive disease course and fibrosarcomatous transformation.
DFSP is a skin tumor that comes from the dermis and is known for its slow growth that spreads into surrounding tissues [16]. It often recurs after surgery but usually doesn't spread to other parts of the body [17]. The way the tumor looks in a clinical setting really depends on how advanced the disease is [17]. At first, it looks pink-to-bluish-red patch on the skin that can be trophic or sclerotic [17]. Over time, it changes into a lumpy nodular mass and eventually becomes an ulcerative, bleeding tumor that sticks out [18]. It feels like it's just on the surface and moves around when touched since it's attached to the skin above, but not to the tissues below [19]. Unfortunately, in the later stage of the tumor, there may be fixation to deeper structures like fascia and muscle [20]. Scalp fixation due to periosteal attachment might happen in the early stages. Telangiectasia can be seen on the surface or around the edges [21].
The tumor grows slowly, so it can take anywhere from weeks to years to develop [21]. It's pretty common for there to be a delay in diagnosis and for the initial lesion to be misdiagnosed, mainly because there aren't any symptoms present [21].
Immunohistochemical staining shows strong positivity for CD34 (sensitivity 84–100%) and vimentin while being negative for CD44, S-100, staining. Factor XIIIa staining. The positivity of the last marker and stromelysin 3 (ST3) is helpful for distinguishing benign fibrous histiocytoma (dermatofibroma) [22]. In our case, Histological analysis showed an infiltrative spindle cell proliferation with storiform architecture and CD34 positivity with adjacent areas of fascicular and herringbone growth pattern and extensive necrosis (fig. 3).
The literature review shows that DFSP rarely metastasizes, and in case of distant spread, it involves the lungs most commonly. As an example, Lombart et al. report a case of recurrent DFSP of the chest wall that metastasized to the lungs and liver [23]. In contrast to our case, metastases from squamous cell carcinomas of the skin usually occur in the pulmonary system, whereas metastases to the pancreas are a highly unusual site. There are also cases similar to Kimmel et al. in which there was a local recurrence of the disease with no distant metastases despite multiple resections and adjuvant radiotherapy in a DFSP with fibrosarcomatous change [24]. Nikolaus et al. also described a case of 45-year-old male with DFSP of the trunk, treated with wide local excision and adjuvant imatinib therapy for PDGFB overexpression [15].
DFSPs have a really strong tendency to invade the nearby tissue. The typical treatment method for this tumor involves a wide and deep local excision (WLE), which also includes the underlying fascia. In a study observing 159 cases, it was found that even though 99% of the patient’s group had complete surgical resection, the pathologic review revealed that only 93 patients (58%) had negative microscopic margins [25]. Lindner et al. found that removing healthy tissues in a circumferential range of 2.5–3.5 cm helped improve local control of the disease [26]. Most people seem to agree that having 3–5 cm of lateral and deep margins is enough for controlling the disease locally.
Conclusion
This case highlights the challenges in surgery for recurrent DFSP with fibrosarcomatous transformation. Pancreatic metastasis, the unique presentation, and the aggressive recurrence pattern underscore the need for vigilant long-term follow-up and consideration for systemic therapies beyond surgery and radiation. Optimal treatment strategies for high-risk DFSP patients needs further studies.
Conflict of interests. The authors declare that there is not conflict of interests.
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
The contribution of the authors. Ashraf ALakkad made a major contribution to the development of the concept of the article with writing and editing the case report. Rawia Mubarak Mohamed agreed to take responsibility for all aspects of the case report. Hazem Muhammad Almasarei was responsible for the radiological analysis and its interpretation in the article. Babitha Alingal Mohammed assisted in the analysis of histopathology images. All the authors approved the final version of the article.
Вклад авторов. Ашраф Альаккад внес основной вклад в разработку концепции статьи, а также написал и отредактировал описание клинического случая. Равия Мубарак Мохамед согласилась взять на себя ответственность за все аспекты представленного описания клинического случая. Хазем Мухаммад Альмасареи отвечал за радиологические исследования и интерпретацию их результатов в представленной статье. Бабита Алингал Мохаммед помогла проанализировать гистологические изображения. Все авторы одобрили финальную версию статьи.
Список литературы доступен на сайте журнала https://klin-razbor.ru/
The list of references is available on the journal‘s website https://klin-razbor.ru/
Информация об авторах
Information about the authors
Равия Мубарак Мохамед – доктор медицины, бакалавр медицины и бакалавр хирургии, член Королевской коллегии патологов Австралии, зав. кафедрой, консультант по патологической анатомии, Burjeel Holdings, ОАЭ. ORCID: 0009-0000-2320-4063
Dr. Rawia Mubarak Mohamed – MD, MBBS, FRCPA Head of Department, Consultant Anatomic Pathology, Burjeel Holdings, UAE. ORCID: 0009-0000-2320-4063
Ашраф Альаккад – доктор медицины, врач-терапевт, каф. внутренних болезней, руководитель программы управления фармакоэпидемиологическими исследованиями, Больница Мадинат Зайед, ОАЭ. ORCID: 0000-0002-4083-2800; Scopus Author ID: 60052817400; Web Of Science Researcher ID: AEW-9201-2022
Dr. Ashraf ALakkad – MD, Internist, Department of Internal Medicine, Chair of Antimicrobial Stewardship Program, Madinat Zayed Hospital, UAE. ORCID: 0000-0002-4083-2800. Scopus Author ID: 60052817400; Web Of Science Researcher ID: AEW-9201-2022
Бабита Алингал Мохаммед – доктор медицины, дипломант Национального совета медицинских экзаменаторов, член Национальной академии медицинских наук, член Королевской коллегии патологов (Великобритания), специалист по патологической анатомии и клинической патологии в медицинском городке Шейха Шахбута (SSMC) в Абу-Даби. ORCID: 0009-0003-3234-0384
Dr. Babitha Alingal Mohammed – MD, D.N.B., M.N.A.M.S., F.R.C.Path. (UK), is an anatomic and clinical pathology specialist at Sheikh Shakhbout Medical City (SSMC) in Abu Dhabi. ORCID: 0009-0003-3234-0384
Хазем Мухаммад Альмасареи – доктор медицины, консультант по диагностической и интервенционной радиологии, кафедра диагностической и интервенционной радиологии, Больница Мадинат Зайед, ОАЭ. ORCID: 0000-0001-9572-3719
Dr. Hazem M. Almasarei – MD, Consultant diagnostic and interventional radiology, Department of diagnostic and interventional radiology, Madinat Zayed Hospital, UAE. ORCID: 0000-0001-9572-3719
Поступила в редакцию: 06.10.2025
Поступила после рецензирования: 14.10.2025
Принята к публикации: 16.10.2025
Received: 06.10.2025
Revised: 14.10.2025
Accepted: 16.10.2025
Список исп. литературыСкрыть список1. Vitiello GA, Lee AY, Berman RSJSC. Dermatofibrosarcoma protuberans: what is this? Surg Clin North Am 2022;102(4):657-65.
2. Lim SX, Ramaiya A, Levell NJ et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol 2023;48(4):297-302.
3. Darier JJADV. Dermatofibromes progressifs et recidivants ou fibrosarcomes de la peau. Ann Dermatol Venereol 1924;5:545-62.
4. Hoffman E. Uber das Knollentreibende fibrosarkom der haut (dermatofibrosarcoma protuberans). Dermatol Z 1925;43:1-28.
5. Bordeaux J, Blitzblau R, Aasi SZ et al. Dermatofibrosarcoma Protuberans, Version 1.2025, NCCN Clinical Practice Guidelines In Oncology. J Natl Compr Canc Netw 2025;23(1).
6. Chicaud M, Frassati-Biaggi A, Kaltenbach S et al. Dermatofibrosarcoma protuberans, fibrosarcomatous variant: a rare tumor in children. Pediatr Dermatol 2021;38(1):217-22.
7. Marcoval J, Moreno-Vílchez C, Torrecilla-Vall-Llosera C et al. Dermatofibrosarcoma Protuberans: A Study of 148 Patients. Dermatology 2024;240(3):487-93.
8. Li Y, Liang J, Xu X et al. Clinicopathological features of fibrosarcomatous dermatofibrosarcoma protuberans and the construction of a back-propagation neural network recognition model. Orphanet J Rare Dis 2021;16:1-9.
9. Jing C, Zhang H, Zhang X, Yu SJDS. Dermatofibrosarcoma protuberans: a clinicopathologic and therapeutic analysis of 254 cases at a single institution. Dermatol Surg 2021;47(2):e26-e30.
10. Chen S, Xiong L, Zhao L, Li Y, Li LJDS. Survival outcomes and prognostic factors of dermatofibrosarcoma protuberans: a population-based retrospective cohort analysis. Current Treatment Options in Oncology. 2022:10.1097.
11. Han Q, Chen M, Yang J et al. Dermatofibrosarcoma protuberans of the face: A clinicopathologic and molecular study of 34 cases. J Dtsch Dermatol Ges 2022;20(11):1463-73.
12. Meng Z, Zhang R, Sun Z et al. Hotspots and future trends of dermatofibrosarcoma protuberans. Front Oncol 2024;14:1399486.
13. Cassalia F, Danese A, Cocchi E et al. Congenital Dermatofibrosarcoma Protuberans – An Update on the Ongoing Diagnostic Challenges. Cancers 2025;17(1):158.
14. Takaoka M, Omatsu J, Awaji K et al. Fibrosarcomatous dermatofibrosarcoma protuberans in a seven-year-old boy. Eur J Dermatol 2021;31(1):106-7.
15. Angouridakis N, Kafas P, Jerjes W et al. Dermatofibrosarcoma protuberans with fibrosarcomatous transformation of the head and neck. Head & neck oncology 2011;3:5.
16. Fields RC, Hameed M, Qin L-X et al. Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann Surg Oncol 2011;18:328-36.
17. Haycox CL, Odland PB, Olbricht SM, Casey BJAops. Dermatofibrosarcoma protuberans (DFSP): growth characteristics based on tumor modeling and a review of cases treated with Mohs micrographic surgery. Ann Plast Surg 1997;38(3):246-51.
18. Ugurel S, Kortmann RD, Mohr P et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)–update 2018. J Dtsch Dermatol Ges 2019;17(6):663-8.
19. Rutkowski P, Klimczak A, Ługowska I et al. Long-term results of treatment of advanced dermatofibrosarcoma protuberans (DFSP) with imatinib mesylate–the impact of fibrosarcomatous transformation. Expert Rev Anticancer Ther 2017;43(6):1134-41.
20. Stacchiotti S, Pantaleo MA, Negri T et al. Efficacy and biological activity of imatinib in metastatic dermatofibrosarcoma protuberans (DFSP). Clin Cancer Res 2016;22(4):837-46.
21. Stacchiotti S, Astolfi A, Gronchi A et al. Evolution of Dermatofibrosarcoma Protuberans to DFSP-Derived Fibrosarcoma: An Event Marked by Epithelial–Mesenchymal Transition–like Process and 22q Loss. Mol Cancer Res 2016;14(9):820-9.
22. Maggoudi D, Vahtsevanos K, Psomaderis K et al. Dermatofibrosarcoma protuberans of the face: report of 2 cases and an overview of the recent literature. J Oral Maxillofac Surg 2006;64(1):140-4.
23. Llombart B, Serra-GuillÈn C, Monteagudo C et al. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Seminars in diagnostic pathology; 2013: Elsevier.
24. Kimmel Z, Ratner D, Kim JY et al. Peripheral excision margins for dermatofibrosarcoma protuberans: a meta-analysis of spatial data. Ann Surg Oncol 2007;14:2113-20.
25. Bowne WB, Antonescu CR, Leung DH et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer 2000;88(12):2711-20.
26. Lindner N, Scarborough M, Powell G et al. Revision surgery in dermatofibrosarcoma protuberans of the trunk and extremities. Eur J Surg Oncol 1999;25(4):392-7.


